

So we have our oxygen, that’s part of our ring here, drawn in the chair conformation. So an iminium ion and then we lose water of course.
#Aldehydy i ketony zadania pdf
LM324 FILETYPE PDF Utlenianie aldehydów za pomocą odczynnika Tollensa All right, so we can think about lone pair of electrons on this alcohol taking this proton, and leaving these electrons behind on this oxygen. So you take that proton and leave those electrons in magenta behind on your nitrogen. So if I follow those electrons, so these electrons in here in blue ended up on our nitrogen to form our imine.
#Aldehydy i ketony zadania plus
So kftony take our generic acid once again so H-A plus and lone pair of electrons on our oxygen could pick up this proton leaving these electrons behind so let’s go ahead and show that. So over here on the right, here I am reflected in the flat silver mirror that I made, and I did this by starting off with a microscope slide which is glass, and then I put that at the bottom of a ml beaker.Īnd so this is the general mechanism to form a hemiacetal. And then two lone pairs of electrons on this oxygen. And so that’s one way to start off your mechanism and of course there is another way to do it.Īnd so you can see that our oxidation state has increased: Shopbop Designer Fashion Brands. So in here, lone pair of electrons on the oxygen, pick up this proton and so forming this bond right here and now you can see we have a good leaving group. Right, attack this carbon push these electrons off onto your oxygen. So we know that in our mechanism we lose one of these protons on our amines so let’s say it’s that proton right there.

So that’s going to give us our final product where we have carbon double bonded to our nitrogen and then we have nitrogen bonded to our Y group here. So this intermediate is called a carbinolamine. So now the carbon has bonded to an oxygen over here on the right, so no longer bonded to a hydrogen, and that’s an oxidation, that’s an oxidation here. That gives the nitrogen a plus one formal charge and the carbon is still bonded to our alkyl groups And so let’s show those electrons here on our nitrogen.

Hopefully you can see how this is one of the possible products and that here we drew it with like a flat plane, and here we’ve drawn it in more of a chair conformation.
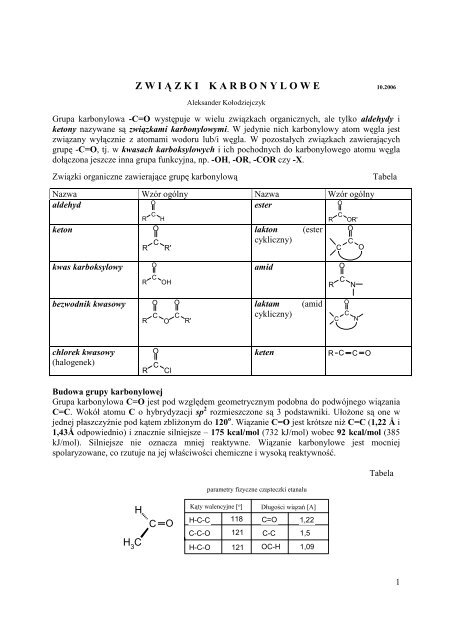
Powstawanie imin i enamin (film) | Khan Academy So these electrons, right, we know that each bond consists of two electrons, so I’m putting in those electrons in here like that. So partial negative oxygen, partial positive carbon this is our electrophile and then a lone pair of electrons on our nitrogen right makes our imine a good nucleophile so they can just attack directly. And so here I have the exact same molecule, except I’ve shown it in a different conformation. This carbon right here will be number one, two, three, four, and five like this. So when we oxidize the aldehydes, we’re going to form a carboxylate anion, and when we oxidize the aldehyde we’re going to reduce the silver. And then if you put some varnish on there you’ll have a really nice silver mirror. And then you can put your ornament on a chemist’s tree. And if we oxidize our alcohol, we’re gonna form a ketone. Here is actually my wife holding the ornament that my students made for us. So let’s go ahead and draw the final product here. Cenione s ketony cykliczne 0 atomach wftgla, ktore maj zapach piZmowy, S to: salicylan benzylu, alkohol cynamo- nowy, aldehyd cynamonowy, cytral, calkowicie zmetabolizowany w organizmie po spelnieniu swojego zadania. izopropyloamina ketones -ketony lead y enie gazu. – l aldehydes -aldehydy aliphatic amines -aminy alifatyczne. W Polsce podobnego zadania podjql sip Polski Komitet Normalizacji. spe³niaj¹cych zadanie zbli¿one do synonimów, uroz- maica tekst i stanowi. Grupy hydroksylowe, estry, ketony b¹dź aldehydy.


 0 kommentar(er)
0 kommentar(er)
